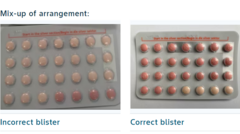
In a significant health alert, Bayer Ltd has initiated a recall of a specific batch of the widely used Yaz Plus contraceptive pills in South Africa following a serious packaging mix-up that raises concerns over their effectiveness. The issue arose when a limited number of blister packs were found to contain 24 inactive pills instead of the standard 24 hormone-containing active pills.
The South Africa Health Products Regulatory Agency collaborated with Bayer on this recall as the company urgently advised women who may have obtained pills from the affected batch (labelled WEW96J, expiring in March 2026) to cease usage immediately. Bayer cautioned that taking the inactive pills could lead to unintended pregnancies, leaving users unaware of the ineffectiveness of their contraception.
Typically, a package of Yaz Plus includes 24 pink active hormone pills followed by four light orange inactive pills. However, customers in this recall may have packs that wrongly include 24 hormone-free pills, jeopardizing contraceptive protection.
Bayer has officially stated that while only a confined number of packs are impacted by this error, all tablets from the recalled batch should not be consumed until users have consulted with a healthcare professional. The company emphasized that even though the problem was limited to a singular batch, they have identified the "root cause" of the packaging error and enforced corrective actions to prevent future occurrences.
Consumers who find themselves in possession of the recalled pills are encouraged to return them to pharmacies for a replacement or a refund. Furthermore, healthcare workers, pharmacists, and medical practitioners that may have stocked the affected products are urged to return them as well.
In light of the situation, Bayer Ltd has established a dedicated helpline for anyone seeking more information and assistance regarding the recall, reaffirming their commitment to women’s health and safety in South Africa.