Peter Marks fears for public health amid rising measles cases and criticizes new HHS Secretary Robert F. Kennedy Jr.'s approach to science.
**Vaccine Expert Resigns Amid Controversy at FDA Over Transparency Issues**

**Vaccine Expert Resigns Amid Controversy at FDA Over Transparency Issues**
A prominent FDA vaccine director resigns, citing conflicts over scientific integrity under new HHS leadership.
In a surprising turn of events, Peter Marks, a high-ranking vaccine official at the U.S. Food and Drug Administration (FDA), has reportedly resigned under pressure. Media outlets have disclosed that Marks, who served as the director of the Center for Biologics Evaluation and Research since 2016, was faced with an ultimatum from Health and Human Services (HHS) Secretary Robert F. Kennedy Jr.: resign or be terminated. In his resignation letter, Marks expressed grave concern about the HHS's current climate, stating, "It has become clear that truth and transparency are not desired by the Secretary," as reported by various sources.
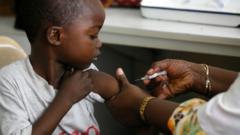
Marks has played a pivotal role in the development of COVID-19 vaccines during the Trump presidency and voiced his apprehension about a resurgent measles outbreak in Texas. The outbreak has claimed two lives so far, with over 523 reported cases nationwide, nearly 400 of which are concentrated in Texas. He warned that such public health crises are a direct consequence of diminishing trust in established scientific principles.
In response to Marks' resignation, the HHS asserted that those unwilling to prioritize scientific integrity under their new leadership were not suited for their roles. Kennedy, who previously promoted vaccine skepticism and misinformation, announced plans to downsize the HHS by cutting 10,000 jobs, affecting various departments including the CDC and FDA. This restructuring is part of his broader agenda to reshape public health policy, raising concerns among many experts and health professionals about the potential impacts on vaccination efforts and public health initiatives in the U.S.