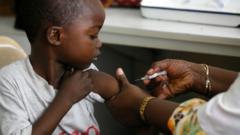
The first malaria treatment suitable for infants and very young children has achieved approval, marking a significant milestone in the fight against one of the deadliest diseases affecting the world's youngest demographic. This new medication is expected to be introduced in African countries within the next few weeks.
Historically, while there were malaria treatments available for children, none were specifically formulated for babies. Prior to this approval, infants were prescribed standard doses for older children, leading to serious risks of overdose. 2023 saw malaria linked to approximately 597,000 deaths globally, with a staggering proportion occurring in Africa; around 75% of these fatalities involved children under five.
The approved drug, produced by Novartis, addresses a long-standing treatment gap for infants weighing less than 4.5kg (approximately 10 lbs). Novartis plans to distribute the medication primarily on a not-for-profit basis, focusing on regions most impacted by malaria. Company CEO Vas Narasimhan lauded the milestone, emphasizing the importance of this development in delivering essential care to the most vulnerable populations.
Known as Coartem Baby or Riamet Baby in various regions, this medication was developed in collaboration with the Medicines for Malaria Venture (MMV), a not-for-profit organization that has received support from multiple governments and global institutions. Several African nations participated in the drug’s assessment and trials, positioning them to be among the first recipients of this crucial treatment.
Martin Fitchet, CEO of MMV, heralded the drug's approval as a pivotal advancement towards curbing malaria's immense toll on health, highlighting its tailored dose for this oft-neglected demographic. Dr. Marvelle Brown, an associate professor at the University of Hertfordshire, reiterated the breakthrough's significance in protecting infants, noting the extremely high mortality rate of malaria among children in sub-Saharan Africa, particularly affecting those with compromised immune systems like infants born with sickle cell disease.
Overall, Novartis’s not-for-profit approach aims to enhance healthcare accessibility and combat existing inequalities, offering new hope in the battle against a disease that has claimed countless lives worldwide.